The federal government has stepped up vigilance within the drug supply chains in the country over the circulation of two confirmed falsified versions of quinine sulphate.
The federal ministry of health said it received a medical alert that the two falsified drugs contain zero active pharmaceutical ingredients.
Boade Akinola, director, media and public relations of the ministry, said the drugs were circulating in West and Central Africa.
Akinola said the two versions of the drug were circulating in Cameroon and Democratic Republic of Congo.
Advertisement
She said the quinine sulphate was used in the treatment of malaria.
She added that the implication of using the falsified one is that it will not be effective and may also lead to other health challenges.
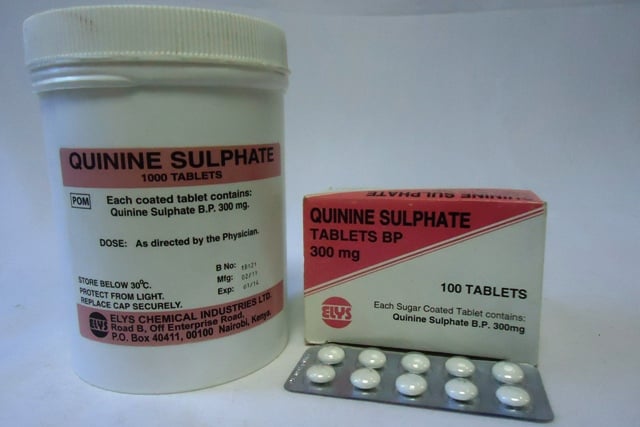
She identified one of the fake products as quinine sulfate 300 mg with 1000 Tablets per container, Batch Number 10H05, expiry date 09/2018, and manufacture date 09/2014.
Advertisement
Akinola said the drug manufactured by Novadina Pharmaceutical Limited, London, United Kingdom was first was discovered in Cameroon.
The director said the other version of the fake drug was quinine sulphate 300mg with 100 tablets per container, batch F4387, expiry date 11/18 and manufacture date 12/14.
She explained that the product was manufactured in India by CAD Pharm, and it was discovered in Bunia, Democratic Republic of the Congo.
She advised Nigerians to be vigilant and report to the nearest National Agency for Food and Drug Administration Control (NAFDAC) office anywhere the drugs were spotted including hospitals and pharmaceutical shops.
Advertisement
The ministry urged Nigerians to also report it the following GSM numbers: +234-8037881120, +234-8055056727 and +234-8035902679.
“If you are in possession of these products, please do not use them,” she said.
“If you have taken this falsified product or if you suffer an adverse effect following its uptake, please seek immediate advice from a qualified healthcare professional and report the incident to NAFDAC.”
Advertisement