First human trial for vaccine against coronavirus begins in the US
BY EJIRO UMUKORO
During an online press briefing, the World Health Organisation said Africa is one of the most unequal societies in terms of production of personal protection kits, access to tools, conducting test trials, lack of ethical systems and capacity, issues of infrastructure, political, legal, and logistical barriers.
Despite these challenges, South Africa and Egypt are among the countries which have begun clinical trials of possible vaccines. Others are Brazil, the USA, and the UK.
In Nigeria, Godwin Emefiele, governor of the Central Bank, tweeted that the bank was ready to offer grants and long term facilities to researchers and organisations who were interested in developing a Nigerian vaccine for COVID-19. An initial sum of USD$20,129 was set aside for this.
Advertisement
There are various types of vaccines being researched. Some of them use the whole coronavirus but in a weakened state, others are using only part of the virus (whether a protein or a fragment) while others are transferring the virus into a different virus that is unlikely or incapable of causing disease. Other vaccines under development are using different pieces of the coronavirus genetic material so that these can be used to stimulate our immune systems.
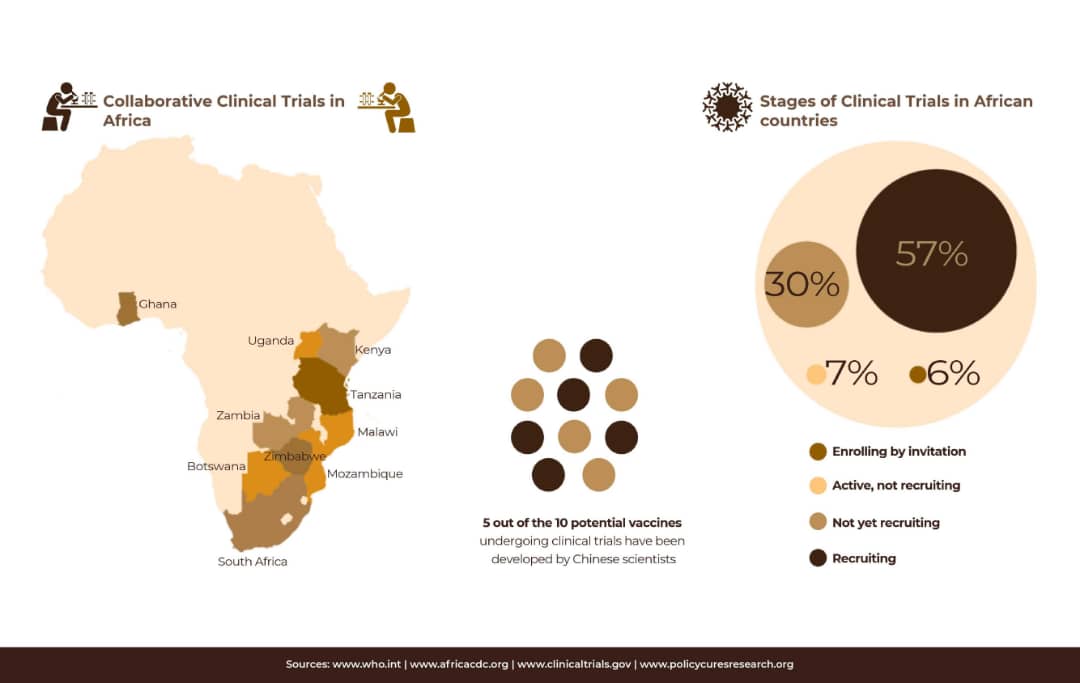
Three African countries, South Africa, Egypt and Ethiopia, have joined the the global COVID-19 research coalition to conduct clinical trials, Pontiano Kaleebu, a Ugandan physician, clinical immunologist, and director MRC/UVRI and LSHTM Uganda Research Unit, advised African countries “to build capacity, engage in more training, exchange of ideas, having ethical systems [to ensure] our vaccines are working, and build trust. Communities will trust scientists if they communicate well.”
Although vaccine trials are nothing new in Africa, Kaleebu emphasised the need for better documentation and data gathering as key to the race for a COVID-19 vaccine cure in Africa.
Advertisement
Matshidiso Moeti, WHO regional director for Africa, said other African countries show the strong capacity to conduct clinical trials and vaccine production include Tunisia, Morocco and Algeria.

Moeti, however, emphasised the need for African citizens to actively participate in clinical trials despite cultural, religious, social, and traditional sensitivities. She debunked the claim that Africans are being used as guinea pigs for COVID-19 clinical trials, saying that clinical trials on the continent are conducted in a good ethical and regulatory processes which have been developed over the years. Populations in countries where clinical trials are being carried have to be informed and should give consent. Countries who participate in clinical trials can expect to receive the vaccine if it is successful.
In July, African Union launched a new consortium bringing together global vaccine developers, funders, and African organisations that conduct clinical trials and providing guidelines that will ensure that adequate data is gathered to ensure the safety and efficacy of any vaccines undergoing clinical trials among African populations.
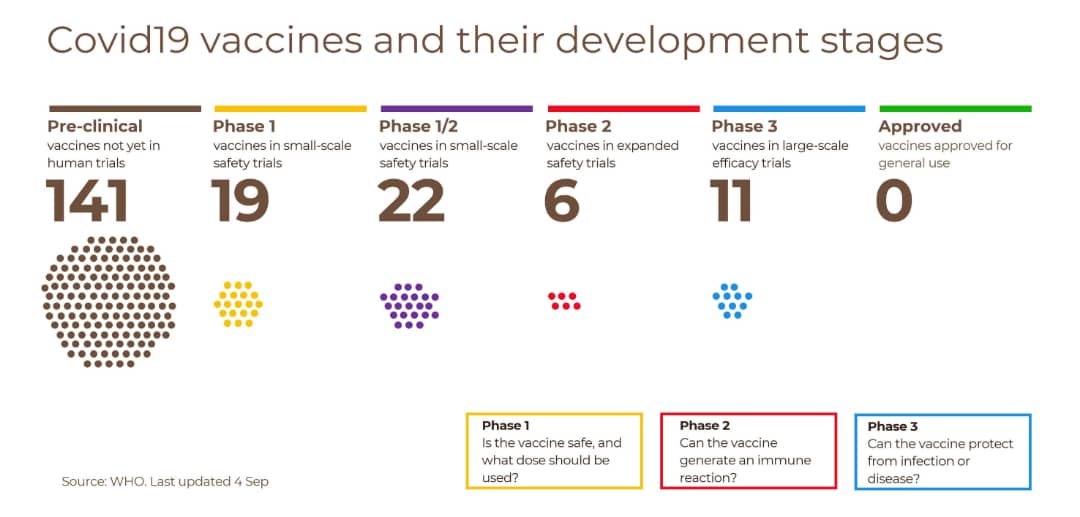 “We need to find out with more precision the efficacy, risks, technologies and products for African people due to different makeup of the different ethnic groups and get relevant information on how it behaves and reacts to this product with African population in African settings in future,” Moeti said.
“We need to find out with more precision the efficacy, risks, technologies and products for African people due to different makeup of the different ethnic groups and get relevant information on how it behaves and reacts to this product with African population in African settings in future,” Moeti said.
Advertisement
According to Tom Kariuki, programme director, African Academy of Sciences, currently, only two percent of clinical trials for all types of vaccines conducted globally occur in Africa. The continent consumes 25% of global consumption of vaccines Vera Songwe, the under-secretary-general and executive secretary of the United Nations Economic Commission for Africam said.
But Moeti encouraged African governments to participate in vaccine clinical trials to ensure the efficacy and safety of future vaccines, including COVID-19 for populations across the continent.
“We want vaccines to be tested in Africa so we understand the safety and we understand the immune response in African populations. It’s important that this product has the opportunity to be tried out in African people. Then we can get the most relevant information about how it behaves, its efficacy, and how people in Africa react to this product. That is why it’s extremely important to carry out clinical trials,” she said.
South Africa, which has so far reported the highest number of Covid-19 infections in the continent, has been testing a vaccine developed by the Oxford Jenner Institute in the UK. The South Africa clinical trial is hosted by the University of Witwatersrand in Johannesburg. This vaccine is also undergoing trials in the UK and Brazil.
Advertisement
Egypt is also conducting clinical trials for a COVID-19 vaccine.

Advertisement
According to Shabir Madhi of South Africa’s Wits University, and principal investigator of Oxford COVID-19 Vaccine Trial in South Africa, “we aim to involve 2000 volunteers. When choosing participants between ages 18 to 65 years for clinical trials, we ensure they are without serious underlying conditions.” The test will also include volunteers with HIV.
He said “the UK has rolled out 8,000 of 10,000 volunteers for its first phase of clinical trials and Brazil plans to enrol 5000.”
In response to increasing cases in urban areas, and concern for an expectedly different impact on African population in towns and villages, the Africa Centre for Diseases Control “has conducted 10 million COVID-19 tests across Africa with one million community health workers deployed and plans to train 100,000 health care workers by the end of 2020 and establish a continent-wide procurement platform for lab and medical supplies,” according to James Ayodele, the Africa CDC principal communication officer.
Advertisement
As the demand for a suitable vaccine continues to grow, Madhi was quick to caution that all studies, irrespective of where they are being conducted, will have to “undergo stringent reviews by local and regulatory authorities and institutions and to have these overseen by independent data and safety monitoring ethics committees.”
Globally 130 candidate vaccine samples have been collated but “only 19 of these have promising preliminary results.”
Advertisement
But will there be a vaccine for all? Madhi dismisses the idea of a publicly available and accessible vaccine.
“Nobody is going to get it for free because it costs to produce it!” he said.
The question of who gets access to a viable vaccine and whether or not it is going to be freely accessible to everyone has been simmering since the search for a cure started.
Ahead of the World Health Assembly in May, world leaders and international organisations signed a letter pledging to work together to ensure that when a safe and effective vaccine is developed, it should be produced rapidly at scale and made available for all countries free of charge.

Among the leaders who signed the pledge are Senegalese President Macky Sall and Ghanaian President Nana Akufo-Addo, former Liberian President Ellen Johnson Sirleaf and former Nigerian President Olusegun Obasanjo.
China, which is one of the countries that is testing a vaccine, has pledged to prioritise and freely supply any Covid-19 vaccines it develops.
And to ensure researchers in Africa have access to vaccine interventions, the WHO has set up the ACT Accelerator, which is an end-to-end effort to hasten the development of vaccines, and its COVID-19 Technology Access Pool. There is also the COVID-19 Global Vaccine Access Facility, headed by Gavi, the Vaccine Alliance, which aims to pay pharmaceutical companies upfront for doses of future vaccines.

The Vaccine alliance will allow for a pooling of resources between high, middle-and low-income countries to allow equitable distribution. Last week, all 54 African countries signed up for the initiative which will see them access at least 200 million doses of the vaccine vaccines that have passed regulatory approval or WHO.
Eight countries that have agreed to self-finance their vaccine doses through the COVAX Facility.
This expression of interest will turn into binding commitments to join the initiative by 18 September, with upfront payments to follow no later than 9 October 2020.
Health workers and other vulnerable populations will be prioritised and then vaccine availability will expand to cover additional priority populations in participating countries.
This report was supported by the Africa Women Journalism Project (AWJP) in partnership with the International Center for Journalists (ICFJ)